The transcriptional control of sRNA DsrA in Enterobacteriaceae
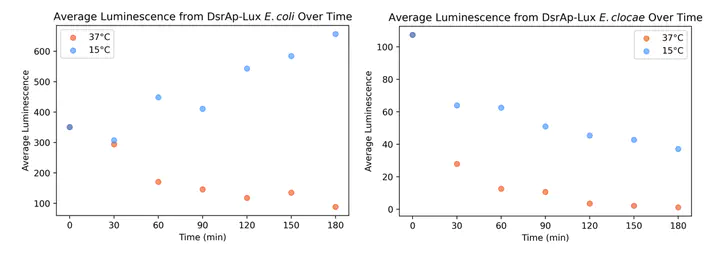 Normalized luminescence by E. coli and E. cloacae dsrAp-luxCDABE-transformants.
Normalized luminescence by E. coli and E. cloacae dsrAp-luxCDABE-transformants.To survive under suboptimal circumstances such as osmotic shift, nutrient starvation, oxidative stress, and extreme temperature, bacteria must respond by adjusting many pathways, which may include activating the repair mechanisms and rewiring metabolic networks. Such general stress response is regulated by the alternative sigma factor RpoS in Enterobacteriaceae. RpoS is regulated at three levels – transcription, translation, post-translation – to respond to stressors efficiently (Hamdallah et al. 2018). During translation, some small RNAs (sRNA) upregulates RpoS by binding to the 5’ untranslated region (UTR) of the rpoS mRNA to open the ribosome binding site (RBS). One sRNA, DsrA, is induced and upregulates RpoS in cold stress in E. coli. In this study, we hypothesized that the DsrA promoter (DsrAp) in some Enterobacteriaceae are not activated in cold stress as some of these species do not have RpoS expressed in cold stress and their DsrAp region differ significantly from that in Escherichia coli. To test this hypothesis, we built dsrAp-luxCDABE reporter constructs in E. coli and Enterobacter cloacae. By measuring luminescence emitted from both E. coli and E. cloacae dsrAp-luxCDABE transformants at 15 °C and 37 °C at 30-minute intervals for 3 hours, we observed that the average luminescence from each E. cloacae transformant cell decreases over time while it increases in the E. coli transformant. It suggests that the E. cloacae DsrAp is not activated at 15 °C. This provides future directions to study the role of DsrA in other Enterobacteriaceae such as Samonella enterica, Citrobacter rodentium, Klebsiella pneumoniae and Serratia marcenscens.